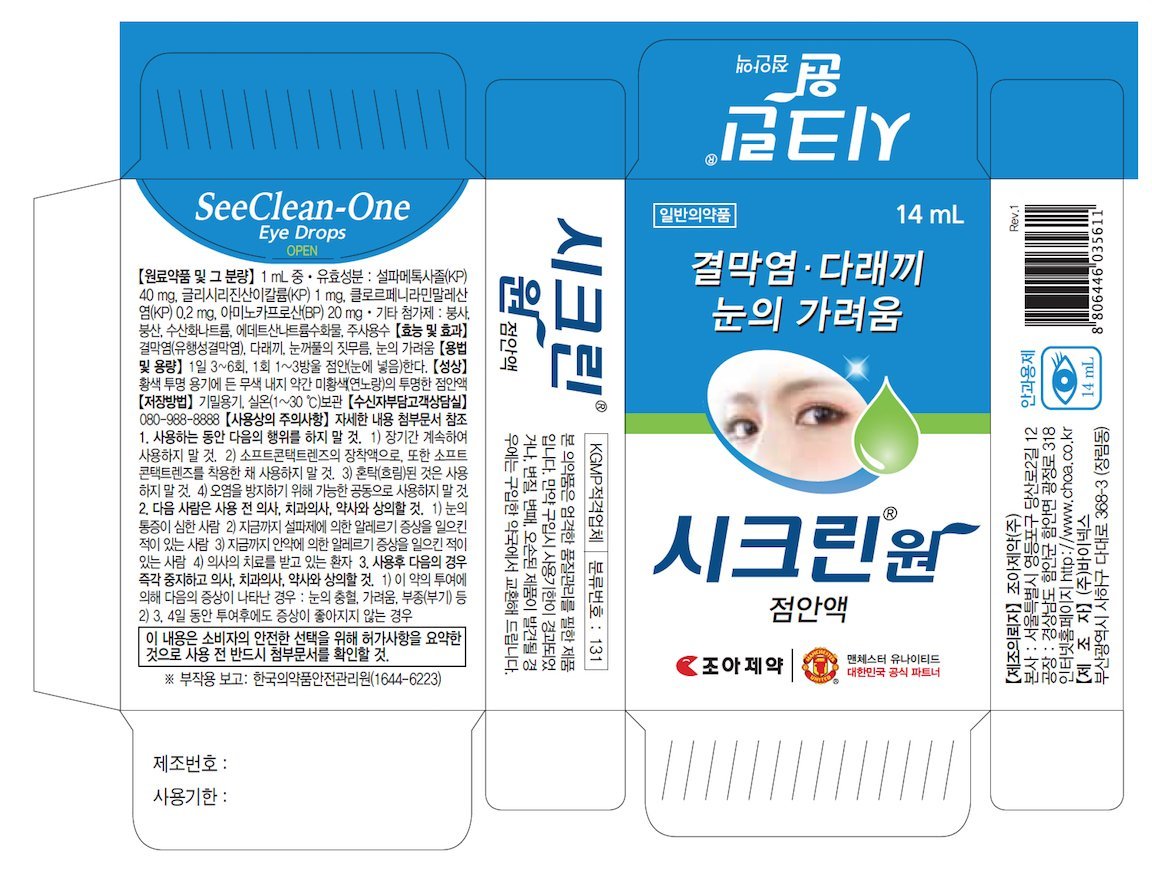
See Clean-One Eye Drops
Dosage form: liquid
Ingredients: BORIC ACID 21.644mg in 14mL
Labeler: Cho-A Pharm.Co.,Ltd.
NDC code: 58354-105
Medically reviewed by Drugs.com. Last updated on Jan 25, 2024.
Boric acid (1.546mg/1ml)
Lubricant
For the temporarily relief of
- burning and irritation due to dryness of the eye
- redness of the eye due to minor eye irritation
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before using
- replace cap after use
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- conditions worsen
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Instill 1-3 drops in the affected eye(s) 3-6 times a day
Sulfamethoxazole, dipotassium glycyrrhizinate, Chlorpheniramine Maleate, Aminocaproic Acid, Disodium Edetate Hydrate, Sodium Tetraborate, Sodium hydroxide, Sterile Water for Injection
| SEE CLEAN-ONE EYE DROPS
boric acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cho-A Pharm.Co.,Ltd. (688056831) |
| Registrant - Cho-A Pharm.Co.,Ltd. (688056831) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cho-A Pharm.Co.,Ltd. | 688056831 | manufacture(58354-105) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.