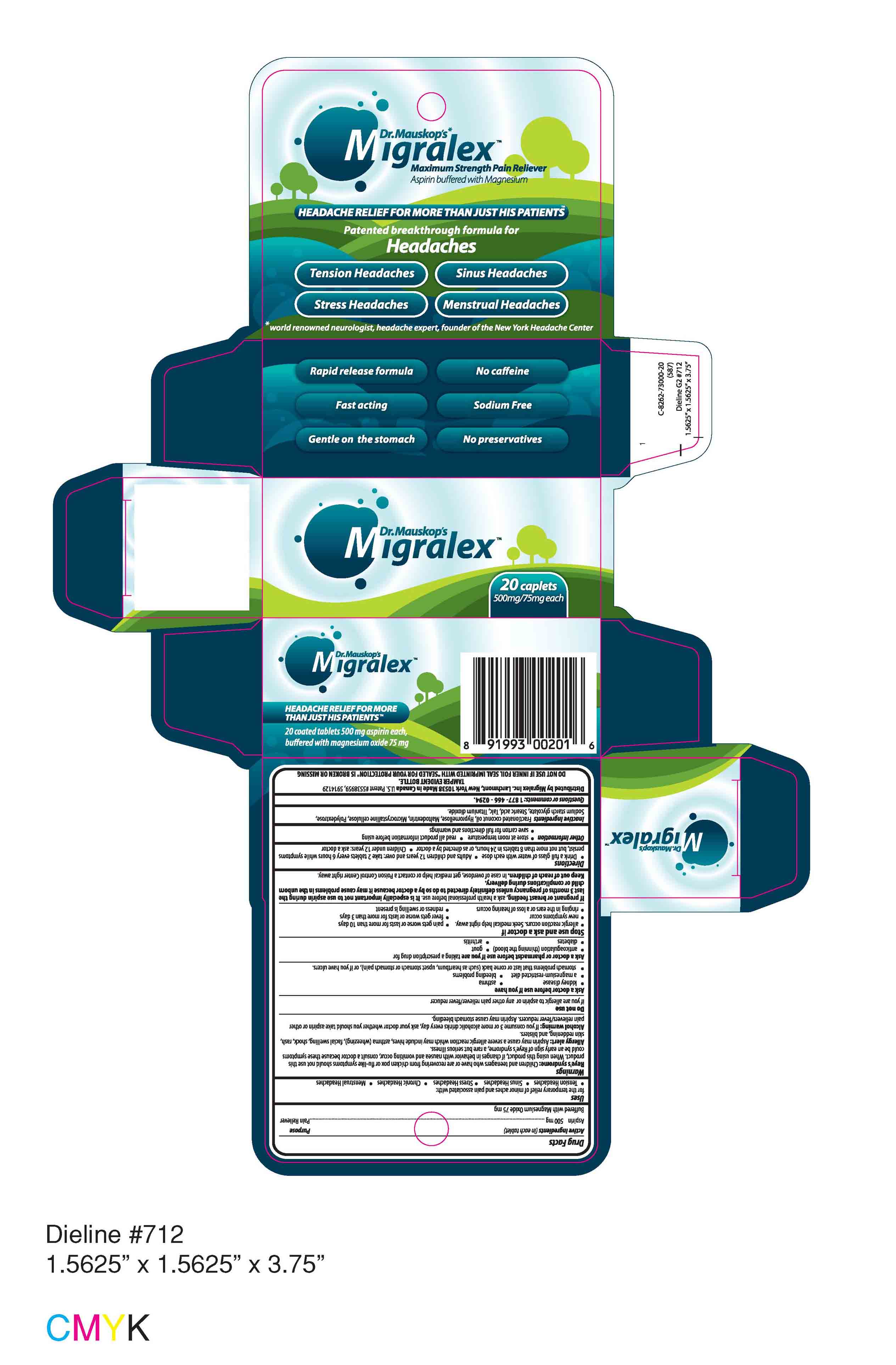
Migralex Dr. Mauskop
Dosage form: tablet, coated
Ingredients: aspirin 500mg, magnesium oxide 75mg
Labeler: Migralex Inc.
NDC code: 49886-001
Medically reviewed by Drugs.com. Last updated on Oct 13, 2023.
Active Ingredients (in each tablet)
Aspirin 500 mg (Purpose: Pain Reliever)
Buffered with Magnesium Oxide 75 mg
Aspirin 500 mg (Purpose: Pain Reliever)
Buffered with Magnesium Oxide 75 mg
Uses
for the temporary relief of minor aches and pain associated with:
for the temporary relief of minor aches and pain associated with:
- Tension Headaches
- Sinus Headaches
- Stress Headaches
- Chronic Headaches
- Menstrual Headaches
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include hives, asthma (wheezing), facial swelling, shock, rash, skin reddening, and blisters.
Alcohol warning: If you consume 3 or more alcoholic drinks everyday, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include hives, asthma (wheezing), facial swelling, shock, rash, skin reddening, and blisters.
Alcohol warning: If you consume 3 or more alcoholic drinks everyday, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
Do not use
if you are allergic to aspirin or any other pain reliever/fever reducer
if you are allergic to aspirin or any other pain reliever/fever reducer
Ask a doctor before use if you have
- kidney disease
- asthma
- a magnesium-restricted diet
- bleeding problems
- stomach problems that last or come back (such as heartburn, upset stomach or stomach pain), or if you have ulcers.
Ask a doctor or pharmacist before use if you are taking a prescription drug for
- anticoagulation (thinning the blood)
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- allergic reaction occurs. Seek medical help right away.
- pain gets worse or lasts for more than 10 days
- new symptoms occur
- fever gets worse or lasts for more than 3 days
- ringing in the ears or a loss of hearing occurs
- redness or swelling is present
If pregnant or breast feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Drink a full glass of water with each dose
- Adults and children 12 years and over: take 2 tablets every 6 hours while symptoms persist, but not more than 8 tablets in 24 hours, or as directed by a doctor
- Children under 12 years: ask a doctor
- store at room temperature
- read all product information before using
- save carton for full directions and warnings
Inactive Ingredients
Fractionated coconut oil, Hypromellose, Maltodextrin, Microcrystalline cellulose, Polydextrose, Sodium starch glycolate, Stearic acid, Talc, Titanium dioxide.
Fractionated coconut oil, Hypromellose, Maltodextrin, Microcrystalline cellulose, Polydextrose, Sodium starch glycolate, Stearic acid, Talc, Titanium dioxide.
Questions or comments: 1 877 - 466 - 0294.
Distributed by Migralex Inc. Larchmont, New York 10538 Made in Canada U.S. Patent #5538959, 5914129
TAMPER EVIDENT BOTTLE.
DO NOT USE IF INNER FOIL SEAL IMPRINTED WITH "SEALED FOR YOUR PROTECTION" IS BROKEN OR MISSING
| MIGRALEX
DR. MAUSKOP
aspirin buffered with magnesium oxide tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Migralex Inc. (009883785) |
Document Id: 0be179e8-6b58-424d-8af2-be237a80bc84
Set id: 31962eb9-690c-4727-852d-d6dfbf842c2a
Version: 1
Effective Time: 20091023
Migralex Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.