Liletta Dosage
Generic name: LEVONORGESTREL 52mg
Dosage form: intrauterine device
Drug classes: Contraceptives, Progestins
Medically reviewed by Drugs.com. Last updated on Jun 29, 2023.
2.1 Dosing Over Time
LILETTA contains 52 mg of levonorgestrel (LNG). Initially, LNG is released in vivo at a rate of approximately 20 mcg/day. This rate decreases progressively to approximately 6.5 mcg/day after 8 years. The average in vivo release rate of LNG is approximately 13.5 mcg/day over a period of 8 years.
For contraception, remove LILETTA by the end of the eighth year. LILETTA can be replaced at the time of removal with a new LILETTA if continued contraceptive protection is desired.
For treatment of heavy menstrual bleeding, replace LILETTA by the end of the fifth year if continued use is needed.
2.2 Timing of Insertion
Refer to Table 1 for instructions on when to start use of LILETTA.
| Starting LILETTA in patients not currently using hormonal or intrauterine contraception |
|
| Switching to LILETTA from an oral, transdermal, or vaginal hormonal contraceptive |
|
| Switching to LILETTA from an injectable progestin contraceptive |
|
| Switching to LILETTA from a contraceptive implant or another IUS |
|
| Inserting LILETTA after pregnancy | |
|
|
|
|
2.3 Insertion Instructions
LILETTA (Figure 1a) is provided in a tray, sealed with a peel-off lid and is inserted into the uterine cavity with the provided inserter (Figure 1b) [see Description (11)] by carefully following the insertion instructions. Do not use if the seal of the sterile package is broken or appears compromised. Use strict aseptic techniques throughout the insertion procedure [see Warnings and Precautions (5.3)]. LILETTA is for single use only.
Note: The inserter provided with LILETTA (see Figure 1b) and the Insertion Instructions in this section are not applicable for immediate insertion after childbirth or second-trimester abortion or miscarriage. For immediate insertion, remove LILETTA from the inserter by pulling LILETTA out of the top of the inserter and insert according to accepted practice.
Figure 1a: LILETTA Intrauterine Contraceptive System (IUS)
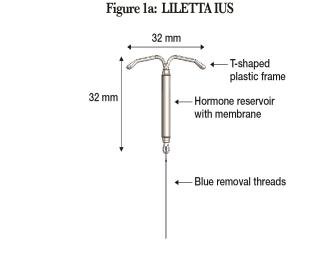
Figure 1b: LILETTA IUS with Inserter
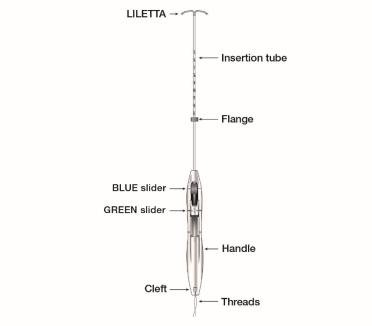
The LILETTA IUS is packaged partially preloaded within the inserter. The threads are passed through the insertion tube and exit through an opening in the handle at the cleft.
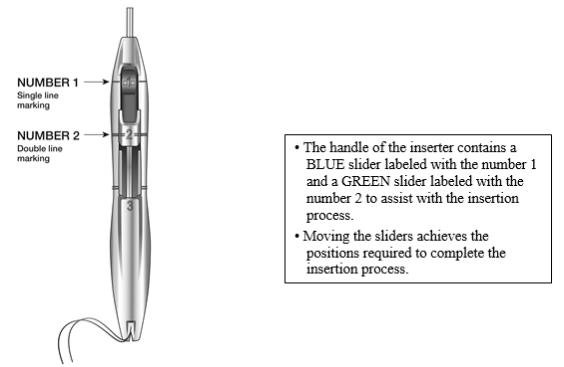
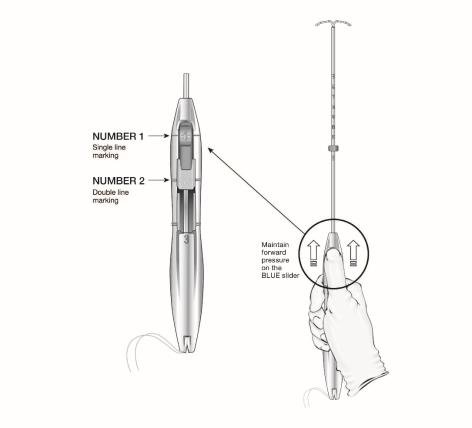
The handle of the inserter contains a BLUE slider labeled with the number 1 and a GREEN slider labeled with the number 2. The handle is labeled with the number 3. The sliders are labeled with the numbers 1 and 2, and the handle is labeled with the number 3 to assist with the insertion process (Figure 2). Moving the sliders achieves the positions required to complete the insertion process.
Figure 2: Inserter Sliders
Planning for Insertion
LILETTA should only be inserted by a trained healthcare professional. Healthcare professionals should become thoroughly familiar with the product, product educational materials, product insertion instructions, prescribing information, and patient labeling before attempting insertion of LILETTA.
Obtain a complete medical and social history to determine conditions that might influence the selection of LILETTA for contraception. If indicated, perform a physical examination and appropriate tests for genital or sexually transmitted infections.1 [See Contraindications (4) and Warnings and Precautions (5.4, 5.10)].
Check the expiration date on the box before opening it. Do not insert LILETTA after the expiration date.
Visually inspect the packaging containing LILETTA to verify that the packaging has not been damaged (e.g., torn, punctured, etc.). If the packaging has any visual damage that could compromise sterility, do not use the unit for insertion [see Warnings and Precautions (5.3)]. Complete the pelvic examination, speculum placement, tenaculum placement, and sounding of the uterus before opening the LILETTA packaging.
Do not open the packaging to insert LILETTA if the following clinical findings occur:
- the cervix is unable to be properly visualized
- the uterus cannot be adequately instrumented (during sounding)
- the uterus sounds to less than 5.5 cm
Preparation for Insertion
Ensure all needed items for LILETTA insertion are readily available:
- Gloves
- Sterile speculum
- Sterile uterine sound
- Sterile tenaculum
- Antiseptic solution
- LILETTA with inserter tray, sealed with a peel-off lid
- Sterile, blunt-tipped scissors
Additional items may be useful:
- Local anesthesia, needle, and syringe
- Sterile os finder and/or cervical dilators
- Ultrasound with abdominal probe
Exclude pregnancy and confirm that there are no other contraindications to the insertion and use of LILETTA.
Follow the insertion instructions exactly as described to ensure proper insertion.
If you encounter cervical stenosis at any time during uterine sounding or LILETTA insertion, use cervical dilators, not force, to overcome resistance. If necessary, dilation, sounding, and insertion may be performed with ultrasound guidance.
Insertion may be associated with some pain and/or bleeding or vasovagal reactions (e.g., diaphoresis, syncope, bradycardia, or seizure), especially in patients with a predisposition to these conditions. Consider administering analgesics prior to insertion.
Use aseptic technique during the entire insertion procedure.
Insertion Procedure
The overall insertion process is conducted in 5 steps.
Step 1 – Preparation of Patient for Insertion
- With the patient comfortably in lithotomy position, do a bimanual exam to establish the size, shape, and position of the uterus and to evaluate any signs of uterine infection.
- Gently insert a speculum to visualize the cervix.
- Thoroughly cleanse the cervix and vagina with antiseptic solution.
- Administer cervical anesthetic, if needed.
- Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity. If the uterus is retroverted, it may be more appropriate to grasp the lower lip of the cervix. Keep the tenaculum in position and maintain gentle traction on the cervix throughout the insertion procedure.
- Carefully sound the uterus to measure its depth.
- The uterus should sound to a depth of at least 5.5 cm. Insertion of LILETTA into a uterine cavity that sounds to less than 5.5 cm may increase the incidence of expulsion, bleeding, pain, perforation, and possibly pregnancy. LILETTA should not be inserted if the uterus sounds to less than 5.5 cm.
- After ascertaining that the patient is appropriate for LILETTA, replace contaminated glove(s) and open the packaging containing LILETTA, noting the lot number.
Step 2 – Opening the Sterile LILETTA Packaging
- Remove the sealed tray containing LILETTA from the box.
- Inspect the sealed tray and do not use the product if the packaging, inserter, or IUS is damaged.
- Lay the tray on a flat surface with the peel-off lid side up.
- Remove peel-off lid.
Step 3 – Loading LILETTA into the Inserter
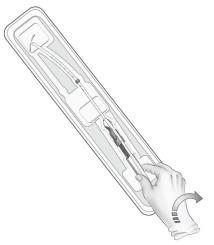
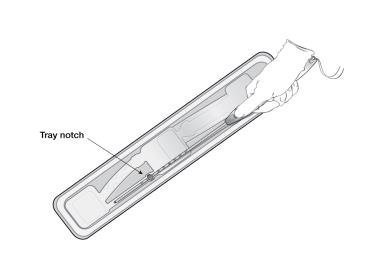
- To remove the inserter from the tray, grasp the handle below the sliders and twist gently (Figure 3).
- NOTE: Do not attempt to remove the inserter by pulling on the tube.
Figure 3: Removing Inserter from Tray
- Ensure both sliders (labeled 1 and 2) are fully forward (Figure 4):
- The BLUE slider (labeled with the number 1) has a single line marking that will align with the handle’s single line marking.
- The GREEN slider (labeled with the number 2) has a double line marking that will align with the handle’s double line marking.
- The BLUE slider (labeled with the number 1) has a single line marking that will align with the handle’s single line marking.
- Grip the handle keeping your thumb or finger in the groove of the BLUE slider (over the numeral 1) and apply forward pressure while ensuring both sliders are fully forward.
Figure 4: Sliders Completely Forward for Loading LILETTA
- Load LILETTA into the inserter:
- Ensure the arms of the IUS are horizontal (aligned to the horizontal plane of the handle and flange); adjust the rotation of the IUS as needed using the flat sterile surface of the tray.
- While maintaining forward pressure on the BLUE slider, gently pull the threads straight back to load LILETTA into the insertion tube. Ensure even tension is applied to both threads when pulling.
- Pull the threads upward or downward to lock the threads into the cleft at the bottom end of the handle (Figure 5); you must lock the threads in the cleft to prevent the IUS from moving out of the top of the insertion tube. Once the threads are locked in the cleft, stop holding the threads.
- After the IUS is loaded, continue to sustain forward pressure on the BLUE slider to maintain a hemispherical dome with the tips of the IUS.
- Ensure the arms of the IUS are horizontal (aligned to the horizontal plane of the handle and flange); adjust the rotation of the IUS as needed using the flat sterile surface of the tray.
- When correctly loaded, the IUS is completely within the insertion tube with the tips of the arms forming a hemispherical dome at the top of the tube (Figure 6).
- If the IUS is not correctly loaded, do not attempt insertion.
- To re-load LILETTA:
▪ Pull the BLUE slider back with your thumb until the groove becomes aligned with the GREEN slider to release the IUS.
▪ Manually pull the threads out of the cleft.
▪ Return the BLUE slider to the forward position and repeat the loading steps.
Figure 5: Locking the Threads in Cleft
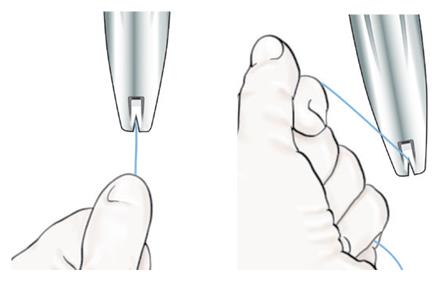
Figure 6: Close-up of Hemispherical Dome at Tip of Tube

- Adjust the flange to the measured uterine depth based on sounding. To adjust, place the flat side of the flange in the tray notch (Figure 7) or against a sterile edge inside of the tray. Slide the insertion tube as necessary to move the flange to the correct measurement. Ensure the flat sides of the flange are in the same horizontal plane as the handle. If, at any step, there is a need to touch the flange or another sterile surface, sterile gloves should be used.
Figure 7: Adjusting the Flange
- If an adjustment to the curvature of the insertion tube is required to accommodate the anatomical orientation of the uterus, you may bend or straighten the insertion tube, but do not touch above the flange unless using sterile gloves. When bending the tube, avoid sharp bends to prevent kinking.
- Once the flange has been properly positioned, avoid contact of flange against objects that can change its position (e.g., tray, speculum, tenaculum, etc.).
Step 4 – Inserting LILETTA into the Uterus
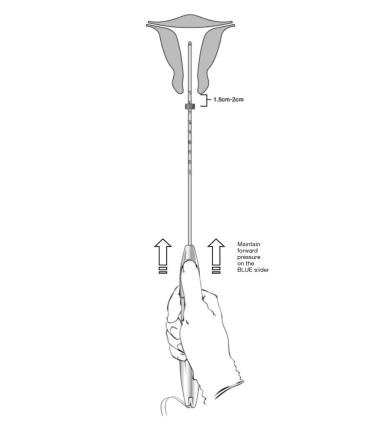
- Apply gentle traction on the tenaculum and continue to apply forward pressure on the BLUE slider while inserting the loaded insertion tube through the cervical os. Advance the tube until the upper edge of the flange is 1.5-2 cm from the external cervical os (Figure 8). Maintain forward pressure on the BLUE slider throughout the insertion process.
○ DO NOT advance flange to the cervix at this time.
○ DO NOT force the inserter. If necessary, dilate the cervical canal.
Figure 8: Advancing Insertion Tube until Flange is 1.5 to 2 cm from the External Cervix
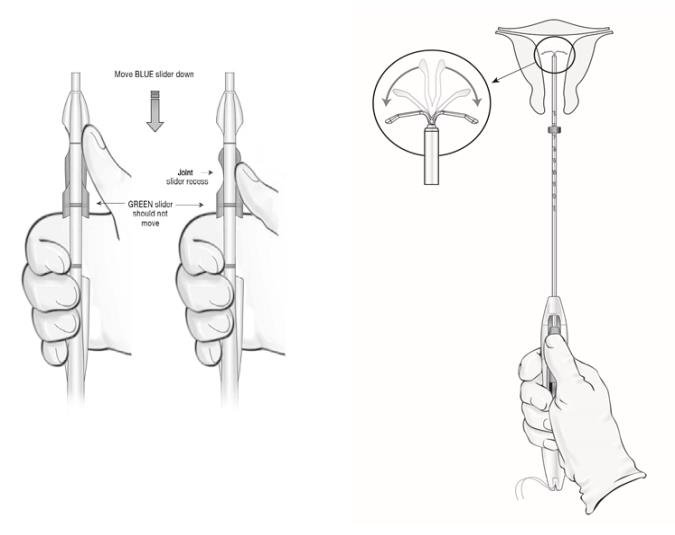
● Using your thumb or finger, gently slide only the BLUE slider back. You will feel slight resistance initially to move the BLUE slider out of its starting position. Continue to move the BLUE slider back until you feel slight resistance again as the BLUE and GREEN sliders will merge together to form a joint slider recess. Do not move the BLUE slider any more than is necessary to create the recess. Maintain the GREEN slider so that the double line markings on the slider and the insertion handle remain aligned (Figure 9). This will allow the IUS arms to open in the lower uterine segment. Do not pull the sliders back any further as this could result in premature release of the IUS at the incorrect location.
Figure 9: Releasing and Opening the Arms of the IUS
- Wait 10-15 seconds to allow for the arms of the IUS to fully open.
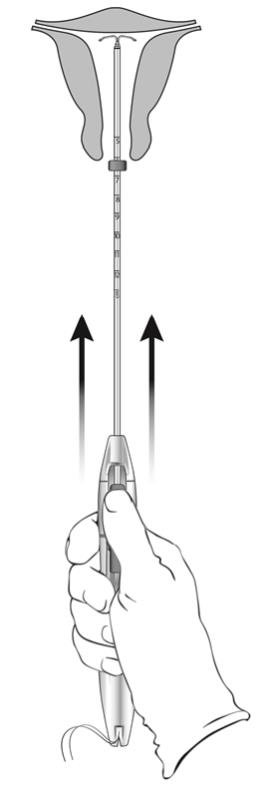
- Without moving the sliders, advance the inserter until the flange touches the cervix. If fundal resistance is encountered, do not continue to advance. LILETTA is now in the fundal position (Figure 10).
- Note: Fundal position is important to prevent expulsions.
Figure 10: Move LILETTA into the Fundal Position
Step 5 – Releasing LILETTA and Procedure Completion
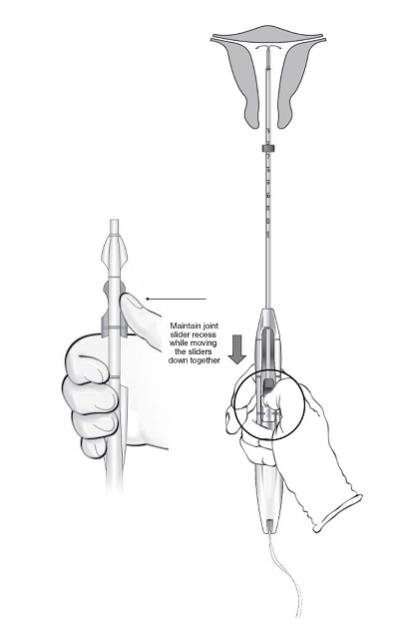
- While holding the inserter steady and maintaining its position relative to the cervix, move both sliders (BLUE and GREEN) together while maintaining the joint slider recess down toward the number 3 on the handle (Figure 11) until a click is heard and the GREEN indicator at the bottom of the handle is visible, signifying deployment (Figure 12).
Figure 11: Releasing LILETTA from the Inserter Tube
- Look at the cleft to ensure the threads were properly released (Figure 12); if not released or if a click is not heard, grasp the threads and gently pull the threads out of the cleft.
Figure 12: Green Indicator Visible and Threads Released from Cleft
- Withdraw the inserter from the uterus.
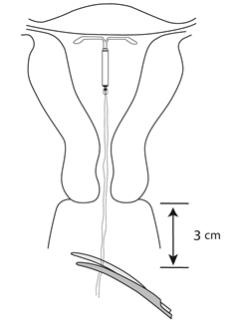
- Use blunt-tipped sharp scissors to cut the IUS threads perpendicular to the thread length, leaving about 3 cm outside of the cervix (Figure 13). Note: Do not cut threads at an angle as this may leave sharp ends.
- Do not apply tension or pull on the threads when cutting to prevent displacing the IUS.
Figure 13: Cut the Threads about 3 cm from the Cervix
 |
- Insertion of LILETTA is now complete.
Important information to consider during or after insertion:
If you suspect the IUS is not in the correct position, conduct the following procedures:
- Check insertion with an ultrasound or other appropriate radiologic test.
- If incorrect insertion is confirmed, remove LILETTA. Do not reinsert the same LILETTA IUS after removal.
Difficult Insertion
If insertion is difficult because the uterus cannot be appropriately instrumented, consider the following measures:
- Use of cervical anesthesia to make sounding and manipulation more tolerable.
- Use of dilators to dilate the cervix if needed to allow passage of the sound or inserter.
- Abdominal ultrasound guidance during dilation and/or insertion.
- If there is clinical concern, exceptional pain, or bleeding during or after insertion, take appropriate steps, such as physical examination and ultrasound, immediately to exclude uterine perforation [see Warnings and Precautions (5.5)].
2.4 Patient Counseling and Record-Keeping
Counsel the patient on what to expect following LILETTA insertion. Discuss expected bleeding patterns with LILETTA use. Review the signs and symptoms associated with infection, perforation, and expulsion that may occur with use of LILETTA [see Patient Counseling Information (17)].
Prescribe analgesics, if indicated.
2.5 Patient Follow-Up
The healthcare professional should consider re-examining and evaluating patients 4 to 6 weeks after insertion and during routine care, or more frequently if clinically indicated. The IUS threads should be checked during each evaluation.
2.6 Removal of LILETTA
Planning and Timing of Removal
If pregnancy is desired, LILETTA can be removed at any time.
If pregnancy is not desired, LILETTA can be removed at any time; however, a contraception method should be started prior to removal of LILETTA [see Dosage and Administration (2.5)]. Counsel patients that they are at risk of pregnancy if they had intercourse in the week prior to removal without use of a backup contraceptive method.
For contraception, LILETTA should be removed after 8 years. LILETTA can be replaced at the time of removal with a new LILETTA if continued contraceptive protection is desired.
For treatment of heavy menstrual bleeding, LILETTA should be replaced at the end of the fifth year if continued treatment is needed.
Preparation for Removal
Ensure all needed items for LILETTA removal are readily available:
- Gloves
- Sterile speculum
- Sterile forceps
Additional items may be required:
- Local anesthetic, needle, and syringe
- Sterile os finder and/or cervical dilators
- Ultrasound with abdominal transducer
- Sterile tenaculum
- Antiseptic solution
- Sterile long, narrow forceps or intrauterine thread retriever
Removal may be associated with some pain and/or bleeding or vasovagal reactions (e.g., syncope, bradycardia, or seizure), especially in patients with a predisposition to these conditions.
After removal of LILETTA, examine the system to ensure that it is intact. The hormone cylinder may slide over and cover the horizontal arms, giving the appearance of missing arms. This does not require further intervention if the system is verified to be intact.
Breakage, embedment in the myometrium, or perforation of LILETTA can make removal difficult [see Warnings and Precautions (5.5)]. IUS breakage may be associated with removal. Analgesia, paracervical anesthesia, cervical dilation, alligator forceps or other grasping instrument, or hysteroscopy may assist in removal.
Removal Procedure
With the patient comfortably in lithotomy position, place a speculum and visualize the cervix.
When the threads of LILETTA are visible:
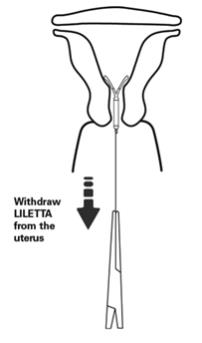
- Remove the IUS by applying gentle traction on the threads with forceps (Figure 14).
- The arms of the device will fold upward as it is withdrawn from the uterus.
- If the IUS cannot be removed with gentle traction on the threads, perform an ultrasound examination to confirm location of the IUS, including assessment for embedment in the myometrium or partial- or total-perforation. If the IUS is in the uterus, use long, narrow forceps to grasp LILETTA. Consider use of a tenaculum, cervical anesthesia, cervical dilators, and/or ultrasound guidance as needed.
- After removal, examine the system to ensure it is intact.
If the threads of LILETTA are not visible:
- Determine location of the IUS and exclude embedment or perforation by ultrasound examination [see Warnings and Precautions (5.10)].
- If the IUS is in the uterine cavity, thoroughly cleanse the cervix and vagina with antiseptic solution. Use a thread retriever to capture the threads or a long, narrow forceps (e.g., Alligator forceps) to grasp LILETTA. Consider use of a tenaculum, cervical anesthesia, cervical dilators, and/or ultrasound guidance as needed. If LILETTA cannot be removed using the above techniques, consider hysteroscopic evaluation for removal.
- If the IUS is not in the uterine cavity, consider an abdominal x-ray or CT scan to evaluate if the IUS is in the abdominal cavity. Consider laparoscopic evaluation for removal, as clinically indicated.
- After removal, examine the system to ensure it is intact.
Figure 14: Removal of LILETTA
2.7 Continuation of Contraception after Removal
If a patient wishes to continue using LILETTA or another intrauterine contraceptive, insertion can occur immediately after removal.
If a patient with regular cycles wants to start a different birth control method, time the removal and initiation of a new method to ensure continuous contraception. Either remove LILETTA during the first 7 days of the menstrual cycle and start the new method or start the new method at least 7 days prior to removing LILETTA if removal is to occur at other times during the cycle.
If a patient with irregular cycles or amenorrhea wants to start a different birth control method, start the new method at least 7 days before LILETTA removal.
If LILETTA is removed but no other contraceptive method has already been started, the new contraceptive method can be started on the day LILETTA is removed. The patient should use a backup barrier method of contraception (e.g., condoms) or abstain from vaginal intercourse for 7 days to prevent pregnancy.
Frequently asked questions
- What are the side effects of IUDs?
- Mirena, Kyleena, Skyla & Liletta - What's the difference?
- Can Plan B make your period late or cause bleeding?
- Can you drink alcohol after taking Plan B?
- How effective is Plan B and how late can you take it?
- What's the weight limit for Plan B?
- How many times can you take Plan B?
More about Liletta (levonorgestrel)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (643)
- Drug images
- Latest FDA alerts (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: contraceptives
- En español
Patient resources
Other brands
Mirena, Plan B One-Step, Kyleena, Skyla, ... +17 more
Professional resources
Other brands
Mirena, Plan B One-Step, Kyleena, Skyla, My Way
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


